Latest News
Latest News
THE OPHTHALMOLOGY MEDICINES COMPANY
Kodiak Sciences is a biopharmaceutical company committed to researching, developing and commercializing next-generation therapies for retinal diseases
OUR MISSION
Kodiak aims to prevent and treat the leading causes of blindness in the developed world

TRAILBLAZING SCIENCE
OUR CREATIVE AND THOUGHTFUL FOUNDATION

“GO-TO” MEDICINES
OUR CHALLENGE TO THE STATUS QUO

SINGULAR FOCUS IN OPHTHALMOLOGY
OUR 24/7/365
OUR PRODUCT CANDIDATES
Three late-phase candidates advancing in parallel, collectively addressing the limitations of today’s therapies across a broad spectrum of retinal diseases
Tarcocimab Tedromer
High-prevalence retinal
vascular diseases
Wet AMD
Diabetic retinopathy
Retinal vein occlusion
Phase 3
KSI-501
High-prevalence retinal
vascular diseases
Indications of interest include wet AMD, diabetic macular edema, retinal vein occlusion and diabetic retinopathy
Phase 3
KSI-101
Macular edema
secondary to inflammation (MESI)
Phase 3
THE ABCD OF OUR SCIENCE
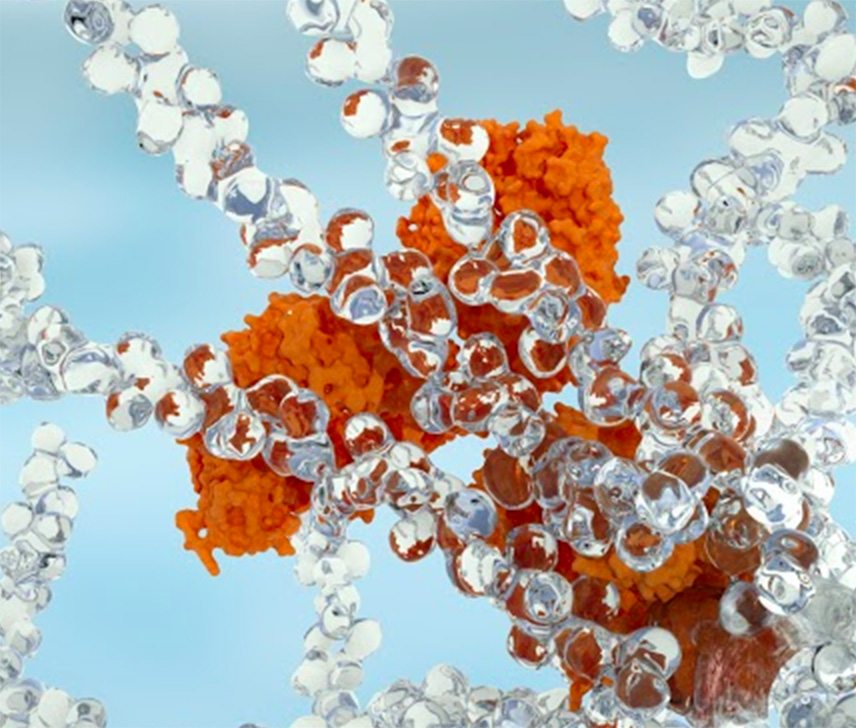
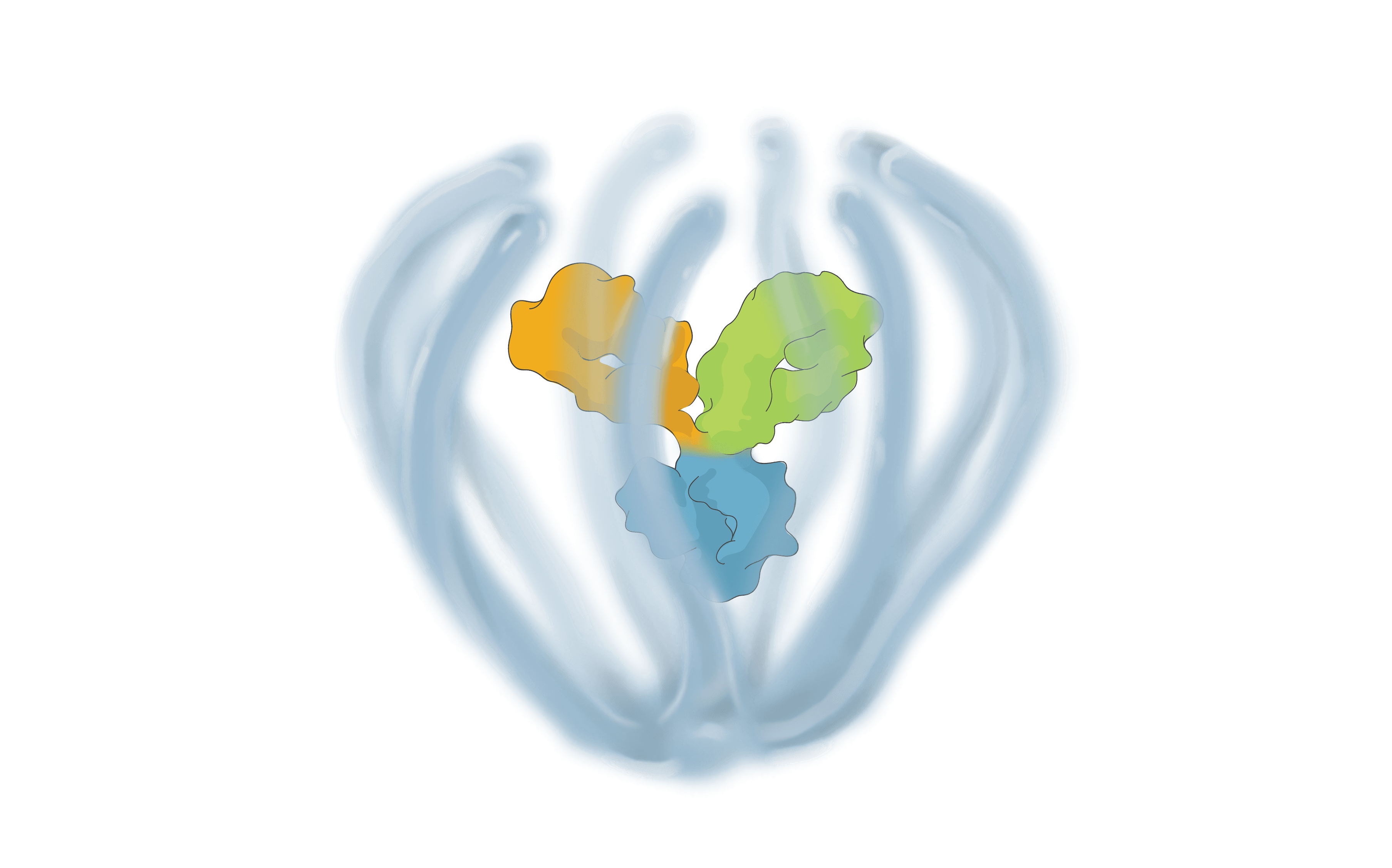
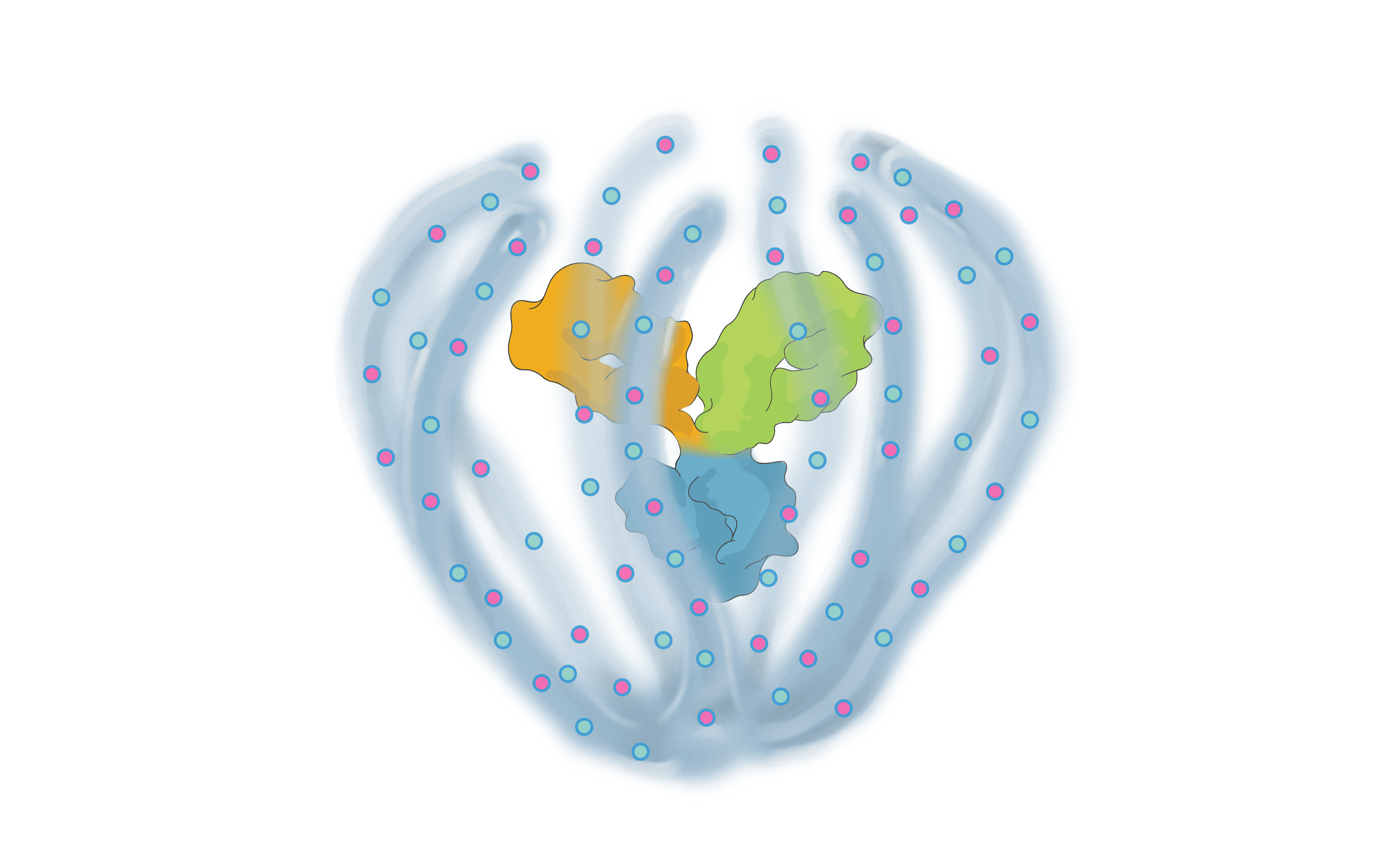
We are bringing new science to the design and development of next generation retinal medicines. Our Antibody Biopolymer Conjugate Drug (ABCD) Platform uses a bio-inspired polymer to enable multi-modal, multi-mechanistic treatment of complex ocular and systemic diseases
Antibody Biopolymer Conjugate Drug
OUR CULTURE AND VALUES
We are actively building a place, our Kodiak Village, where we are inspired, where we feel valued, and where we can grow our personal and professional journeys while advancing our mission. Historically referred to within Kodiak as the 4 C’s, our values are:

CURIOSITY
Staying fresh.
Asking why.
Being naturally inquisitive.

CREATIVITY
A safe place to think outside the box.
A focus on how to solve the problem.

COMPASSION
For ourselves.
For our colleagues.
For our patients.

COURAGE
Saying no when that’s the right answer.
Saying yes when that’s the right answer.
Regardless.
WORKING AT KODIAK
We aspire to global leadership in ophthalmology through internal focus and by aggregating top talent, technologies, discoveries and ideas.